In this issue:
EPA Regulation Review
Method 533
New QEC 1000mL Packer
Drinking Water Grants
Pittcon Award
Glass is a Liquid?


The US Environmental Protection Agency has begun a formal review-and-comment process that may lead to the regulation of PFOA and PFOS in drinking water.
On March 10, 2020 the EPA published its “Announcement of Preliminary Regulatory Determinations for Contaminants on the Fourth Drinking Water Contaminant Candidate List” in the Federal Register, opening a 60-day public comment period. That is the first step on a very long road that could result in the federal regulation of these two PFAS compounds in drinking water.
Specifically, the announcement addresses eight of the compounds listed in the fourth Contaminant Candidate List (CCL 4). The EPA is making preliminary determinations to regulate two contaminants (PFOS and PFOA) and to not regulate the other six contaminants (1,1-dichloroethane, acetochlor, methyl bromide, metolachlor, nitrobenzene, and RDX).
If the EPA finalizes these preliminary regulatory determinations, it would represent the beginning of the Agency's regulatory development process, not the end. As required by SDWA, the EPA seeks comment on these preliminary determinations and is asking for information and comment on other per- and polyfluoroalkyl substances (PFAS) and potential regulatory approaches.
The EPA is also presenting an update on strontium (from the third regulatory determination) and two other CCL 4 contaminants for which the Agency is not making preliminary determinations (1,4-dioxane and 1,2,3-trichloropropane).
Public comments will be accepted through May 11, 2020, although several organizations have requested a 30-day extension of the comment period. To read the full text of the EPA announcement as well as the comments submitted, please follow this link.
In December 2019 the US EPA announced the new validated Method 533 for testing additional short chain per- and polyfluoroalkyl substances (PFAS) in drinking water.
Method 533 measures PFAS by isotope dilution anion exchange solid phase extraction and liquid chromatography/tandem mass spectrometry (LC-MS/MS). The lowest concentration minimum reporting levels (LCMRLs) for the method analytes range from 1.4 to 16 parts per trillion (ppt).
The combination of this new method and existing EPA methods can now measure 29 different PFAS compounds in drinking water. For more information, visit EPA’s website and view Method 533. A table of analytes for EPA Methods 533 and 537.1 is available on QEC’s website.
QEC's UltraLab™ 250mL polypropylene container with mated leak-proof polypropylene closure is the ideal choice for PFAS sample collection. Available Custom-Preserved™ for either Method 533 or Method 537.1 applications. Please contact your QEC representative or our customer service team for more information.
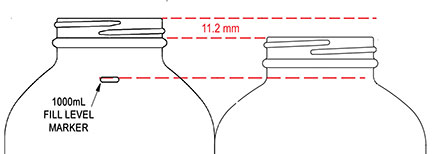
Introducing QEC's new 1000mL amber packer with a raised fill marker. This latest addition to QEC’s packer line is made of Type III amber glass for excellent UV protection with a 53-400 finish, and available with a number of closure options.
The slightly taller form factor (compared to our 32 oz packer) allows for significantly more head space when holding its nominal 1000mL capacity (as indicated by the fill marker).
This means easier access to the contents in the laboratory with less likelihood of spillage. For more information on QEC’s new packer, please contact your QEC representative or our customer service team (1-800-255-3950).


Pennsylvania, Maryland, West Virginia, Delaware, and the District of Columbia are receiving a total of $2,882,000 in grants from the US EPA to test for lead in school drinking water.
According to a series of announcements made on April 1, Pennsylvania will receive a $1.7 million grant, Maryland $513,000, West Virginia $262,000, Delaware $209,000, and the District of Columbia $158,000.
The funding will be used to support voluntary testing for lead in drinking water at schools and child care centers. Grantees will assist schools in implementing lead in drinking water testing including identifying sources of lead such as fountains.
Testing results carried out using grant funds must be made publicly available. The funds are for testing only and do not address remediation.

Quality Environmental Containers is honored to have been one of the handful of exhibitors chosen to receive the Standout Exhibitor award from the 2020 Pittcon Conference and Expo.
QEC was one of only two awardees in the "Smart Exhibit Access and Attendee Navigation" category. In the language of the citation:
"The exhibit was striking with their use of branded colors, but the way they displayed their products and engaged attendees was so very well organized, that it easily allowed visitors to navigate to areas of interest. A great way to display a lot of products in a fun, colorful, and engaging way!"
QEC has been exhibiting at Pittcon for 25 years, and it's always been about "displaying our wares"-- letting visitors see and examine the quality of our containers for themselves. This has always been a successful part of our marketing, and we very much appreciate Pittcon for recognizing our efforts.

It's one of those annoying little bits of "scientific trivia" that's just not true. But where did the idea come from?
In medieval European cathedrals, the glass panes sometimes are thicker at the bottom than they are at the top, giving the appearance that the seemingly solid glass has melted. This observation influenced internet trivia sites and even high school chemistry teachers to say that glass is actually a supercooled liquid.
Glass, however, is actually neither a liquid nor a solid. It is an amorphous solid—a state somewhere between those two states of matter.
Solids are highly organized structures. They include crystals, like sugar and salt, with their millions of atoms lined up in a row. Glasses, though more organized than liquids, do not attain the rigid order of crystals. Amorphous means the atoms don’t exhibit that long-range order.
When glass is made, the material is quickly cooled from its liquid state but does not solidify when its temperature drops below its melting point. At this stage, the material is a supercooled liquid, an intermediate state between liquid and glass. To become an amorphous solid, the material is cooled further, below the glass-transition temperature. Past this point, the molecular movement of the material's atoms has slowed nearly to a stop and the material is now a glass. For practical purposes, glass is like a solid although a disorganized one.
Over long periods of time, the molecules making up the glass shift themselves to settle into a more stable, crystal-like formation. The closer the glass is to its glass-transition temperature, the more it shifts; the further away from that changeover point, the slower its molecules move and the more solid it seems.
Note to cathedral tour guides everywhere: Some medieval glass panes are thicker on the bottom due to how the glass was made. At that time, glassblowers flattened glass cylinders to make panes of glass. The panes often were not uniformly flat and workers installing the windows preferred, for whatever reason, to put the thicker sides of the pane at the bottom.
Source: Scientific American, February 2007
® 2020 Quality Environmental Containers, Inc. • info@qecusa.com